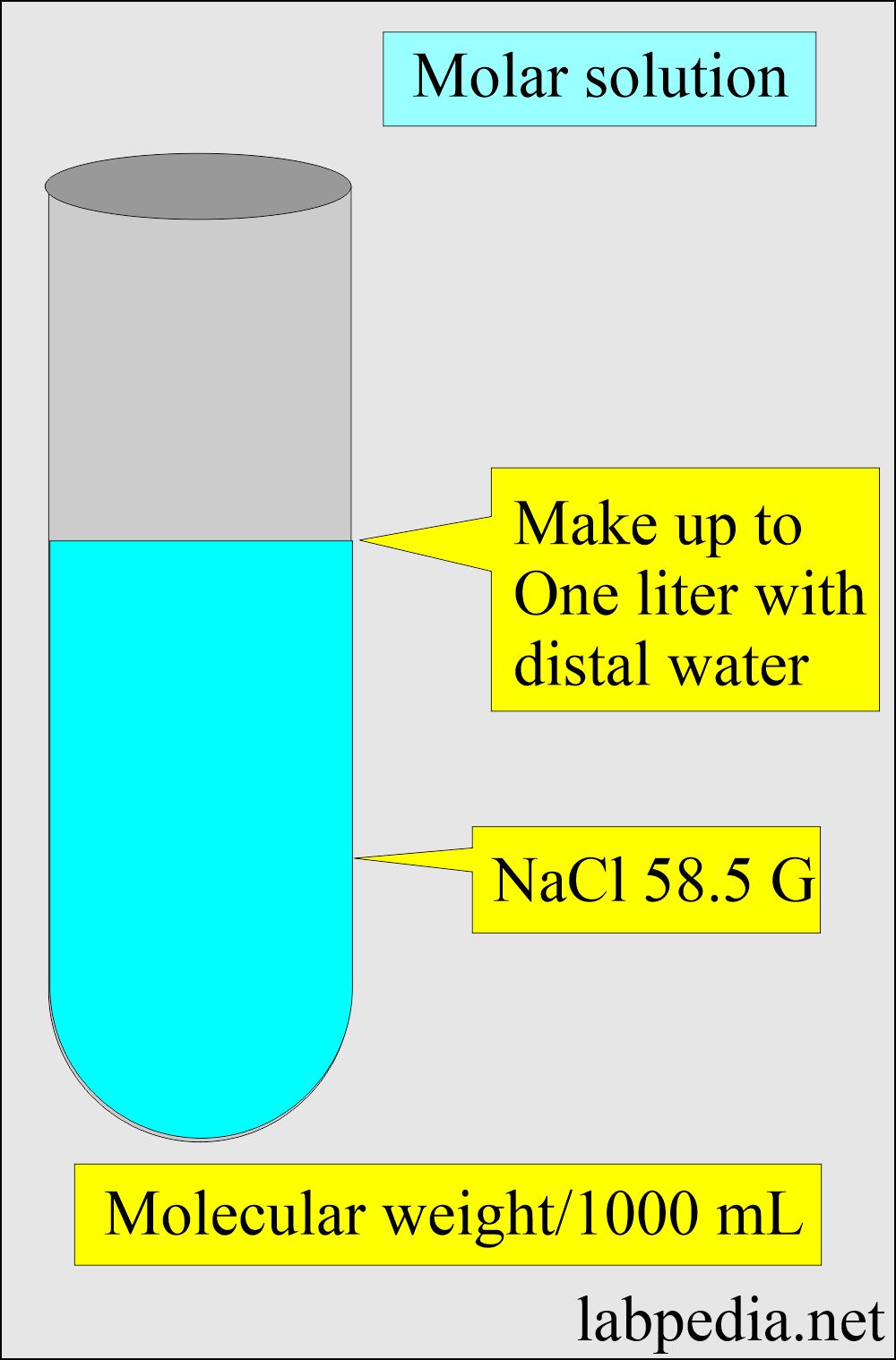
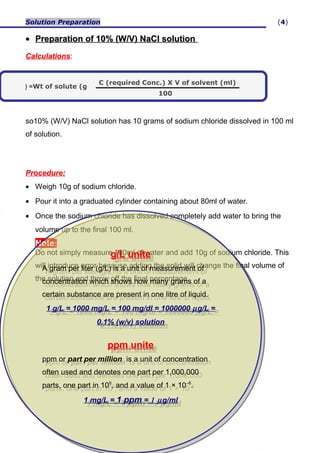
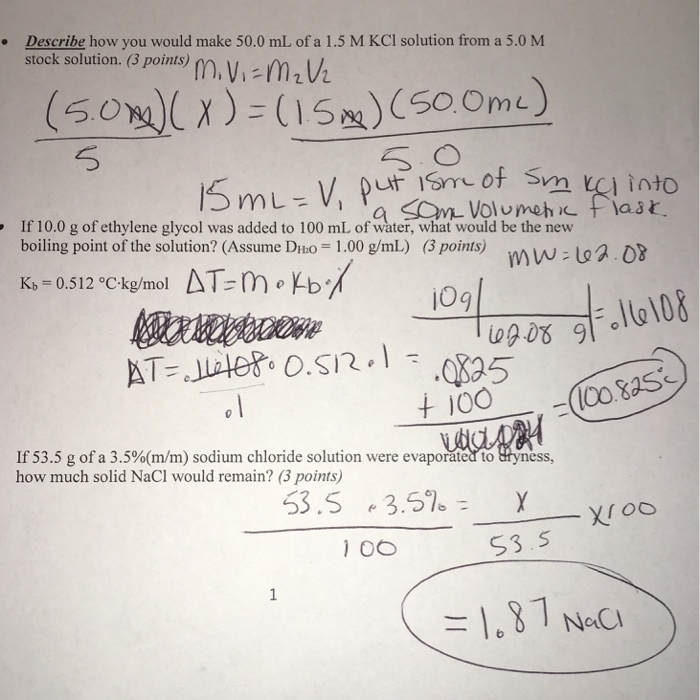Calculate the molarity of `KCl` solution prepared by dissolving `7.45 g` of `KCl` in `500 mL` of... - YouTube
57. If 0.2 M aq. solution of KCI is isotonic with 0.2 MK,SO at same temperature then van't Hoff factorof K2SO4 is(1) 0.2(2) 0.5(3) 0.8(4) 0.3