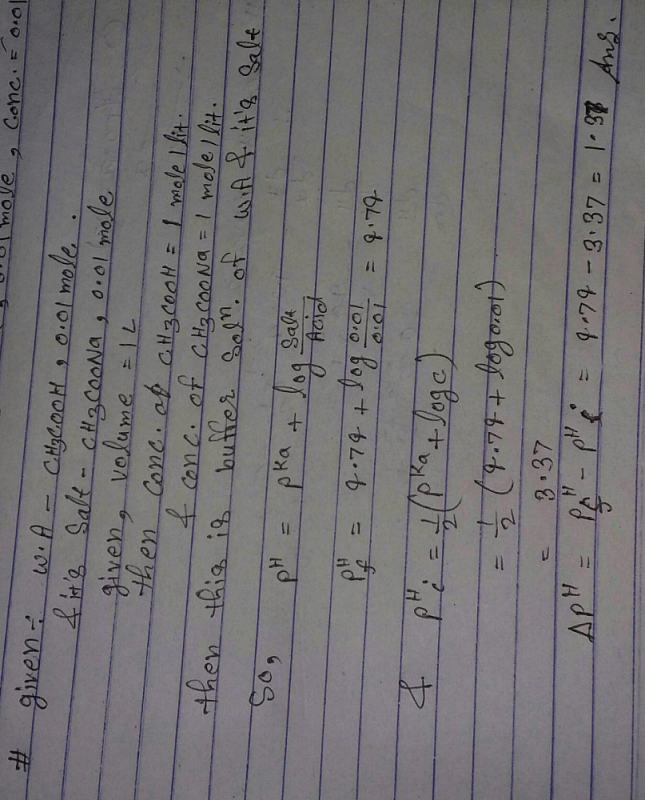![SOLVED: Calculate the concentrations ofall molecular and ionic species,and the pH in aqueous solutions that have the following compositions: 0.01M acetic acid (CH3COOH), Ka= 1.74x10-5 (give [CH:COOH], [CH:COo:], [H+],and pH) 0.05M acetic SOLVED: Calculate the concentrations ofall molecular and ionic species,and the pH in aqueous solutions that have the following compositions: 0.01M acetic acid (CH3COOH), Ka= 1.74x10-5 (give [CH:COOH], [CH:COo:], [H+],and pH) 0.05M acetic](https://cdn.numerade.com/ask_images/d6e81f711fc2493883d79db57ed94a51.jpg)
SOLVED: Calculate the concentrations ofall molecular and ionic species,and the pH in aqueous solutions that have the following compositions: 0.01M acetic acid (CH3COOH), Ka= 1.74x10-5 (give [CH:COOH], [CH:COo:], [H+],and pH) 0.05M acetic
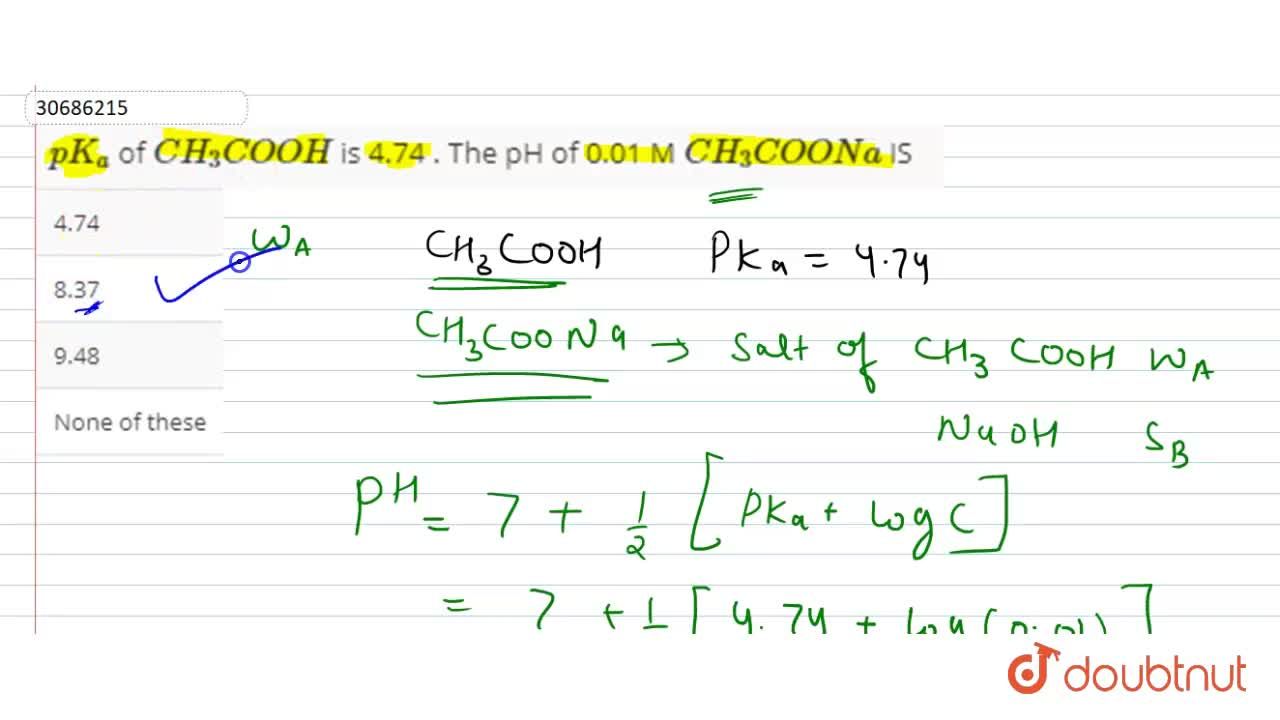
![The `[H^(+)]` of a resulting solution that is 0.01 M acetic acid `(K_(a)=1.8xx10^(-5))` and 0.01... - YouTube The `[H^(+)]` of a resulting solution that is 0.01 M acetic acid `(K_(a)=1.8xx10^(-5))` and 0.01... - YouTube](https://i.ytimg.com/vi/6n7yj8M7Dp4/maxresdefault.jpg)
The `[H^(+)]` of a resulting solution that is 0.01 M acetic acid `(K_(a)=1.8xx10^(-5))` and 0.01... - YouTube
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://haygot.s3.amazonaws.com/questions/1938773_1231298_ans_7f19b0e0d221405fbf38b0df680608aa.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]
Calculate the pH of a 0.01 M solution of acetic acid. Ka for CH3COOH is 1.8 x 10^-5 at 25°C. - Sarthaks eConnect | Largest Online Education Community
![The P^(H) of 0.01 M solution of acetic acid is 5.0 What are the values of [H^(+)] and Ka respectively ? The P^(H) of 0.01 M solution of acetic acid is 5.0 What are the values of [H^(+)] and Ka respectively ?](https://d10lpgp6xz60nq.cloudfront.net/ss/web/577303.jpg)
The P^(H) of 0.01 M solution of acetic acid is 5.0 What are the values of [H^(+)] and Ka respectively ?

SOLVED: A 40.0 ml sample of 0.01 M ethanoic acid (CH3COOH) is titrated with 0.02 M sodium hydroxide, NaOH. Calculate the pH after adding 30.0 ml of NaOH solution. *Given Ka of